The third in the Industrial HVAC Design series will address some of the key IAQ/IEQ considerations in the biotechnology, pharmaceutical & advanced technology manufacturing industries.
Learning Objectives
- Understand design guidelines specific to biotechnology and pharmaceutical manufacturing facilities.
- Understand design guidelines specific to advanced technology manufacturing facilities.
- Identify measures that need to be taken to protect manufacturing processes in advanced technology facilities.
Air quality design insights
- Specialized biotechnology, pharmaceutical and advance technology manufacturing facilities have stringent sterility and purity requirements compared to other industrial manufacturing facilities.
- Contamination from airborne particulates is a significant concern in these facilities.
- The facility should be designed and operated with cascading air quality, achieved through proper air classification and air pressurization. This helps protect the most critical zone preventing the infiltration of contaminated air from adjacent areas of lower air quality.
Industrial facilities encompass a wide range of sectors including food, beverage, life sciences and advanced technology manufacturing. In this this third installment, readers will understand more about both the generic principles and specific aspects of Indoor Air Quality – Indoor Environmental Quality (IAQ-IEQ) design in industrial life sciences and advanced technology manufacturing facilities
Biotechnology and pharmaceutical manufacturing facilities add another layer of complexity to basic design considerations in an industrial setting because of more stringent requirements in the sterility of the production environment. The number and size of airborne particulates and specifically microbial particles, are the main sources of contamination in the production process.
Depending on the drug being produced or biologic process being run, the International Standards Organization (ISO) Classification of particulate matter in the production space must be used to establish certain requirements of sterility and purity that the production environment must possess. The drugs should only be produced in the designated ISO class or in a better class once those requirements.
Design guidelines for biotechnology and pharmaceutical manufacturing facilities

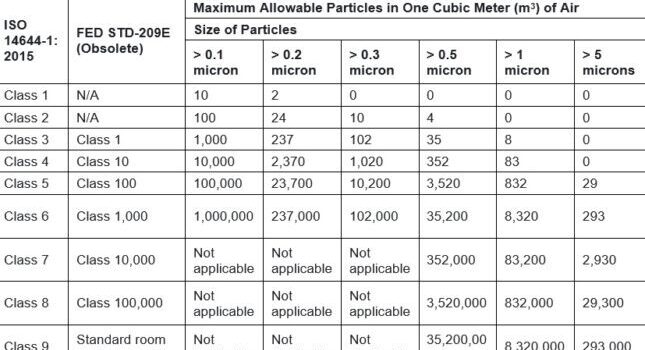
ISO 14644, is the international standard for the design of cleanrooms, replacing the U.S. General Service Administration’s FED-STD 209 E standard. However, this standard may still be found in many legacy documents. Table 1 provides the new ISO classification specifications with side-by-side comparison of the obsolete FED-STD 209E standard.
Facilities with clean room production should be designed and operated with cascading air quality (by proper air classification and air pressurization) to protect the zone with most stringent ISO air quality. Similar to hygienic zoning, flow of materials, personnel and air should be designed to prevent the infiltration of contaminated air from adjacent areas of lower air quality and transition.
The drug production area, the most critical area, should go through certain qualification testing to comply with the specifications and applicable current good manufacturing processes (cGMP) regulations before usage, depending on the criticality and cleanliness requirements of the drug/equipment being produced. These facilities require the high care spaces to be as concise and confined as possible because the air exchange rates in these spaces are incredibly high at 30-50 air changes per hour (ACH). As such, the highest care spaces are typically rooms inside of larger rooms with cascading higher ISO classifications the closer you are to the production zone.
The air filtration system is also an integral part of the pharmaceutical and biotechnology design and operation. Filters, including prefilters and particulate final filters, such as high-efficiency particulate air (HEPA) filters or ultralow particulate air (ULPA) filters, should always provide appropriate air quality. This requires them to regularly monitored for pressure drops and replaced to supply the required air quality and quantity.
Measures must be taken to prevent and eliminate the presence of dangerous particulates in air being transferred from production areas to nonproduction areas. Where air is contaminated with biohazards, adequate exhaust systems must be utilized to isolate and expel the contaminated air to the outdoors to maintain an acceptable level of sterility and occupant safety. Even with the most stringent purity requirements, it is understood that there will always be a certain level of contaminants. The goal is to minimize to an acceptable threshold, not to eliminate.
Other critical parameters to be maintained at their specified level in the cleanroom area are temperature and humidity. Higher humidity supports microbial growth whereas too little humidity can cause problem with static electricity. Facilities central heating, ventilation and air conditioning system should be actively monitored and controlled to maintain the desired levels of temperature and humidity in the outer zone along with dedicated, high air exchange, high filtration air within the cleanroom.
The discharge air entering the production space passes through a final HEPA or ULPA filter to ensure virtually no contaminants in the ductwork can get into the sterile production space. Within the space, the airflow is uniform, laminar flow once through and removed with minimal opportunity to recirculate in the space.
Dedicated, redundant temperature, humidity and differential pressure monitoring and control systems are preferred in the high care zones with redundant data signal pathways to the central facility industrial controls and automation system. Controls systems should include necessary built-in alarms and action sequences in cases of deviations from the desired parameters within the high-care production or lower-care nonproduction and support areas.
Design considerations in advanced technology manufacturing facilities
Advanced technology fabrication spaces are fairly similar to high sterility bio-pharma production spaces. They all operate in the similar ISO Class environments with the most stringent environmental requirements closest to the final production space. In addition to exceptional particulate, temperature and humidity control, these facilities also have stringent vibration controls. They are typically tightly isolated fabrication rooms within a larger production module with support functions inside of a much larger facility housing multiple such production modules.
Additionally, airflow distribution, including return paths and temperature stratification profiles are of critical concern. These considerations typically require significant computational modeling to ensure proper flow patterns, be they laminar or turbulent, which are often complicated by the large array of fan filter units required. Advanced technology facilities with cleanroom design frequently employ computational fluid dynamics models to evaluate pressure, temperature and air velocity profiles that balance the temperature, humidity and particulate controls relative to production staff positioning and movement. Specific process production equipment and conveyor belts outside of their tightly controlled microenvironment enclosures are also included in the models.
The key points presented in this article should serve as a good starting point in understanding some of the unique design considerations for IAQ-IEQ for life sciences and advanced technology manufacturing facilities.



