Purdue University biomedical engineers have developed a handheld paper device that could accurately detect COVID-19 and other coronaviruses with the right manufacturing process, but developing the process is expensive.
Only a select number of state and local laboratories have permission from U.S. health officials to use diagnostic tests for COVID-19, a coronavirus-caused disease. If the virus is spreading nationwide, most communities do not have access to the necessary tests.
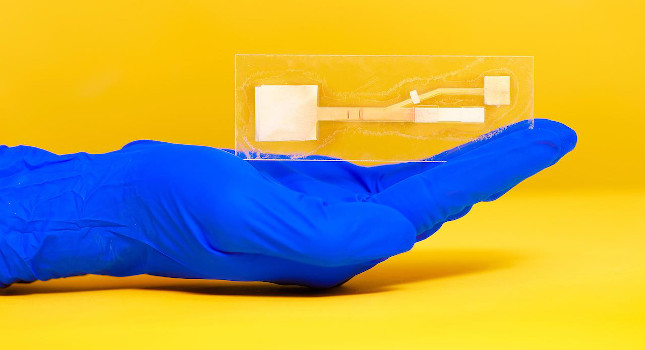
Purdue University biomedical engineers have developed a handheld paper device that quickly and accurately detects a different strain of coronavirus, MERS-CoV, even in really small quantities. A clear test result can be read directly from the device itself, making it portable.
Because the device isn’t specific to any virus, the same platform could be used to detect the COVID-19 strain. But developing a process to manufacture it would cost at least a couple of million dollars.
“Paper-based devices are already manufactured – pregnancy tests are paper-based. Because this device has a more complex shape, a process hasn’t been developed to make it available on a commercial scale. However, many processes in electronics and paper manufacturing could be translated to scaling up this device,” said Jacqueline Linnes, Purdue’s Marta E. Gross Assistant Professor of Biomedical Engineering.
Linnes specializes in building portable diagnostic tools that can rapidly detect a range of infectious diseases.
Her lab’s devices are made out of cheap but robust paper-like materials, such as glass fiber and cellulose, that have been demonstrated to detect HIV and cholera. To know if a sample is positive, a user just looks for a second line to appear next to a control line on the device’s paper strip – similar to reading a pregnancy test.
So far, Linnes’ team has just been able to produce these devices on a lab scale, which calls for cutting out the paper components by hand.
“The most difficult aspect of producing this device is definitely the assembly,” said K Byers, a Ph.D. candidate in biomedical engineering at Purdue.
These challenges may easily be overcome with existing manufacturing techniques, the researchers said. The paper device could be produced using the same roll-to-roll manufacturing tools that make pregnancy tests and a “pick-n-place” process, which is already used to build electronics.
The device format wouldn’t need to change in order to detect other diseases. As the device scales up, however, it would also need to be more sensitive to detect a lower concentration of a virus for clinical relevance.
“This paper device isn’t dependent on a particular virus or sequence. To detect COVID-19, we would just need an assay design specific to that sequence, which could come from a nasal or throat swap sample. Just like with MERS-CoV, a user could load the assay with liquid into the paper platform, fold the device and let it run,” Linnes said.
Folding the device automatically completes a multistep process needed for detecting a virus. When the device folds over, a liquid wash and chemical substances called reagents push the assay up a paper strip to make an easily visible detection line.
A user can check the strip within 40 minutes to see if the sample tested positive.
Because the reagents are already dried onto the device, a hospital worker who might need to test someone outside of a facility wouldn’t have to carry around reagent vials. The worker would simply need a couple of test tubes to prepare the assay.
– Edited by Chris Vavra, associate editor, Control Engineering, CFE Media and Technology, [email protected].



